Manufacturing & Pathogen Safety
Octapharma Has Pioneered a Number of Safety Innovations That Have Set the Standard for the Protein Products Industry
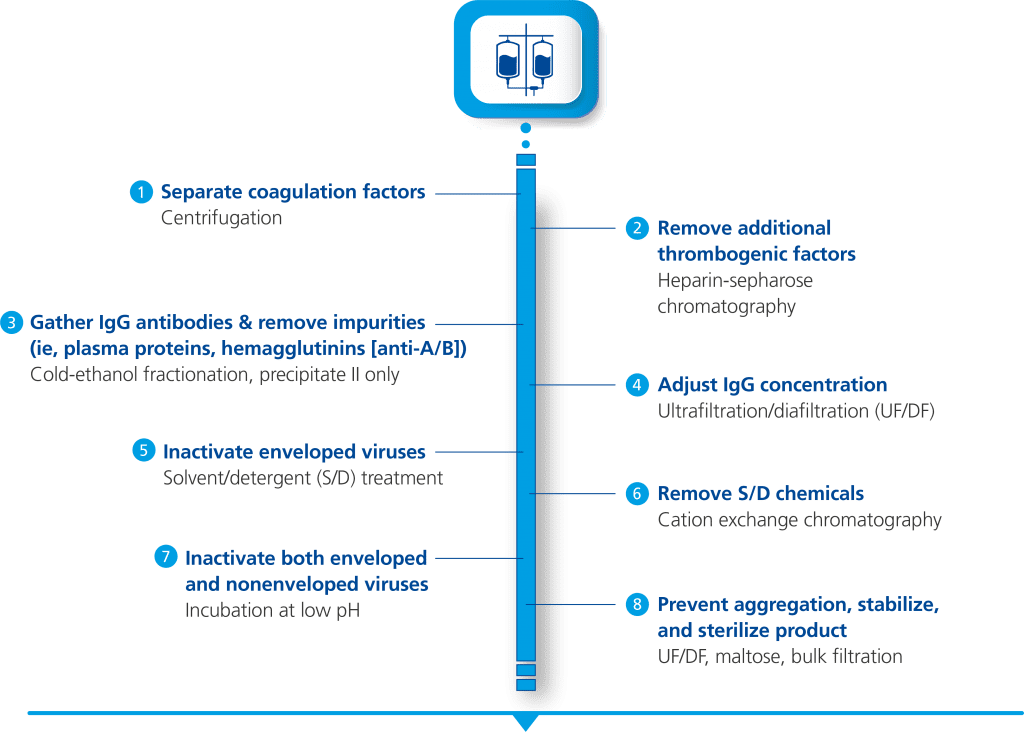
Octagam 10% therapy for DM manufacturing and purification process1-3
Octagam 10% contains all 4 subclasses of IgG, with a percentage distribution equivalent to the one found in normal plasma
- Octagam 10% is made from large pools of donated plasma which go through a rigorous purification process for the inactivation and removal of viruses
- Other precautions against viral transmission include: selection of plasma donors, screening of donations and plasma pool, as well as final product testing for viruses.
- Octagam 10% is manufactured by cold ethanol fractionation followed by ultrafiltration and chromatography. The manufacturing process includes treatment with a solvent/detergent, and incubation at low pH
Validated Viral Removal and Inactivation3-5
The manufacturing process for octagam 10% therapy for adult dermatomyositis has been validated for its capacity to remove and inactivate both enveloped and nonenveloped viruses
All units of human plasma used in the manufacture of octagam 10% liquid are provided by FDA-approved blood establishments only, and are tested by FDA-licensed serological tests for HBsAg, antibodies to HCV and HIV and Nucleic Acid Test (NAT) for HCV and HIV-1 and found to be non-reactive (negative).
*Not calculated for global log reduction factor (LRF).
HBsAg=hepatitis B surface antigen; HCV=hepatitis C virus; HIV-1=human immunodeficiency virus-1; MEV=mouse encephalomyelitis virus; PPV=porcine parvovirus; PRV=pseudorabies virus; SBV=sindbis virus.
Octapharma was the first company to apply S/D viral inactivation in routine production. The S/D process, in conjunction with other critical steps in broader viral inactivation, has delivered a proven record of no known viral transmissions for 3 decades.2
References:
- Octagam 10% Full Prescribing Information. Paramus, NJ: Octapharma; rev March 2022.
- Octapharma. Data on file.
- Buchacher A, Kaar W. Intravenous immunoglobin G from human plasma—purification concepts and important quality criteria. In: Bertolini J, Goss N, Curling J, eds. Production of Plasma Proteins for Therapeutic Use. Hoboken, NJ: John Wiley & Sons, Inc; 2013.
- Dichtelmüller HO, Biesert L, Fabbrizzi F, et al. Transfusion. 2011;51(7):1412-1430.
- Dichtelmüller HO, Biesert L, Fabbrizzi F, et al. Transfusion. 2009;49(9):1931-1943.